Determination of an Equilibrium Constant Lab
INTRODUCTION Through use of the Flow-Measuring Apparatus this experiment is designed to accustom students to typical methods of measuring the discharge of an essentially incompressible fluid whilst at the same time giving applications of the Steady-Flow Energy. In this case the formation constant K f.

Solved Report Sheet Experiment Colorimetric 22 Determination Chegg Com
Michael Evans Georgia Institute of Technology.
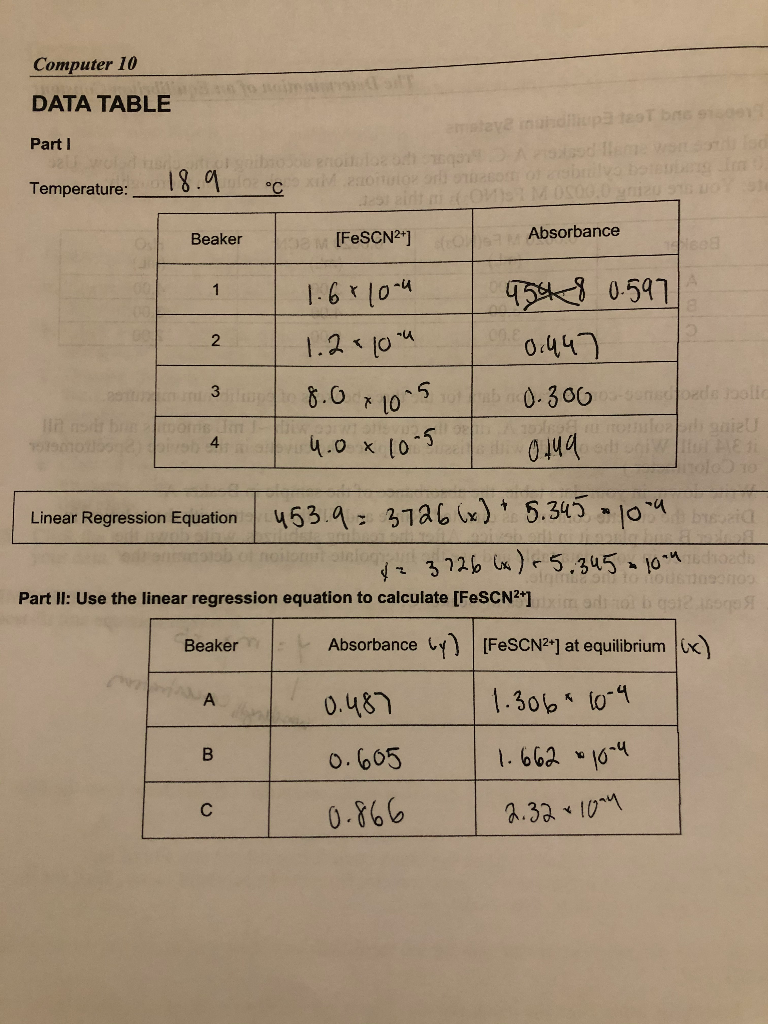
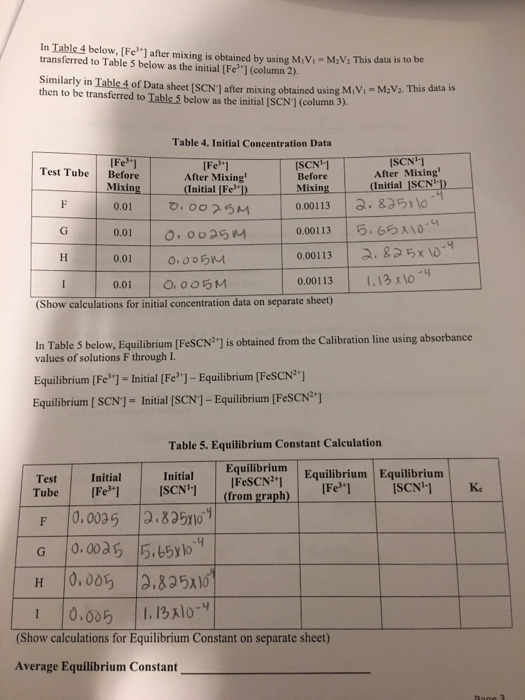
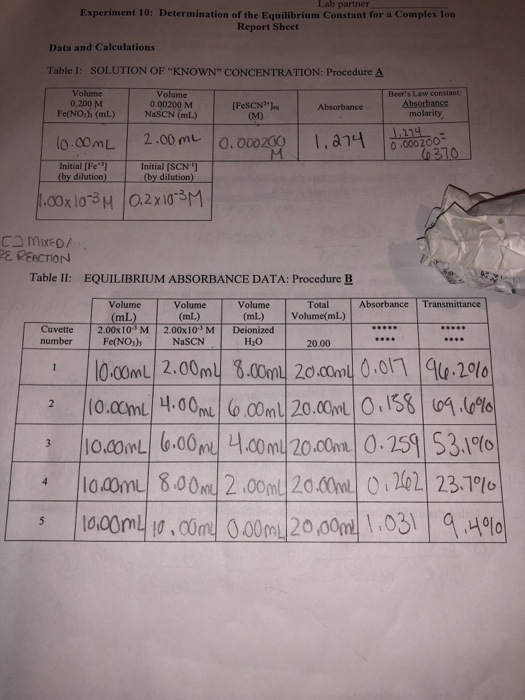
. Where A eq and A. FeSCN2 and to see if the constant is indeed the same under different. Kf FeNCS 2eq Fe 3eq NCS eq 5 To completely characterize this reaction it is necessary to know the value for K f.
The purpose of this experiment is to determine the equilibrium constant for the reaction. The equilibrium constant K for a chemical system is the ratio of product concentrations to reactant concentrations at equilibrium each raised to the power of their respective stoichiometric coefficientsMeasurement of K involves determination of these concentrations for systems in. What is the purpose of the determination of an equilibrium constant lab.
How do you calculate FeSCN2 EQ. DETERMINATION OF DISCHARGE AND HEAD LOSS USING A FLOW-MEASURING APPARATUS. FeSCN 2 eq is calculated using the formula.
Equilibrium constant of the reaction. K f can be calculated through an experimental determination of the equilibrium concentration of the.

Solved The Determination Of An Equilibrium Constant Post Lab Chegg Com
Solved Determination Of Equilibrium Constant Lab Chegg Com
Solved Lab Partner Experiment 10 Determination Of The Chegg Com
Comments
Post a Comment